Fast-Tracking Safe Drugs without Sacrificing Scientific Rigor – Article by Alex Tabarrok
Here’s how to do it
In a post earlier this year, I noted that Japan has significantly liberalized its approval process for regenerative medicine. Writing in Forbes, Bart Madden and Nobelist Vernon Smith outline a similar proposal for the United States.
Recently, Japanese legislation has implemented the core Free To Choose Medicine (FTCM) principles of allowing not-yet-approved drugs to be sold after safety and early efficacy has been demonstrated; in addition, observational data gathered for up to seven years from initial launch will be used to determine if formal drug approval is granted. …
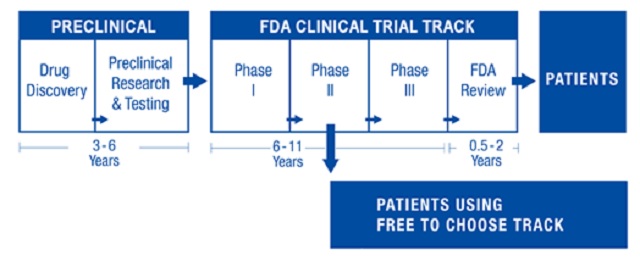
FTCM legislation in the U.S. would create a dual track system (see figure below) that preserves the existing FDA clinical trial process while offering patients an alternative. Patients, advised by their doctors, would be able to contract with a drug developer to use not-yet-approved drugs after Phase I safety trials are successfully completed and one or more Phase II trials have demonstrated continued safety and initial efficacy.
The resulting early access could make FTCM drugs available up to seven years before conventional FDA approval, which entails Phase III randomized control trials and a lengthy FDA review before the FDA makes an approval decision. …
The heart of the dual track system is the Tradeoff Evaluation Drug Database (TEDD) which would be available to the public through a government-supervised web portal. TEDD would contain all treatment results of FTCM drugs including patients’ health characteristics and relevant biomarkers, but no personal identification.
This open access database would be a treasure-trove of information to aid drug developers in making better R&D decisions consistent with fast-paced learning and innovation. …
Today’s world of accelerating medical advancements is ushering in an age of personalized medicine in which patients’ unique genetic makeup and biomarkers will increasingly lead to customized therapies in which samples are inherently small. This calls for a fast-learning, adaptable FTCM environment for generating new data.
In sharp contrast, the status quo FDA environment provides a yes/no approval decision based on statistical tests for an average patient, i.e., a one-size-fits-all drug approval process.
I hold the Bartley J. Madden Chair in Economics at the Mercatus Center, so I am biased, but this is an important proposal. Japan is leading the way and similar ideas are being discussed in Great Britain, but as the most important pharmaceutical market in the world, the United States has an outsize influence on world drug development. We need to lower costs and speed new drugs to market.
This post first appeared at Marginal Revolution.
Alex Tabarrok is a professor of economics at George Mason University. He blogs at Marginal Revolution with Tyler Cowen.